A candle flame
What’s a combustion reaction?
When two molecules bump into each other under the right circumstances, they may exchange electrons in ways that change both molecules into new kinds of molecules.
What’s a molecule?
More about electrons
All our chemistry articles
Releasing heat or light or electricity
While they’re doing that – reacting to each other – they may also release some electrical energy in the form of heat or light. This is what happens whenever there is a fire.
Electrical energy
What is heat?
Photons and light
An example of a combustion reaction
The earliest fires were stars, where when hydrogen atoms meet under a lot of pressure from gravity, they merge together into helium atoms and let off some extra energy – that’s the sunshine we get from our Sun.
What is a star?
More about hydrogen
All our space articles
A lot more examples
The same kind of thing happens in a forest fire, or when you light a candle or a match. Candles are made of hydrocarbon molecules (sometimes oil, sometimes beeswax, sometimes tallow from animal fat), and matches are made from hydrocarbon molecules (wood). When they get hot enough, these hydrocarbon molecules react with the oxygen in the air.
Hydrocarbons
More about beeswax
What is friction?
Thunder and lightning
The heat can come from friction, like when you strike a match, or from another fire, like when you hold the match to the candle, or from lightning that starts a forest fire, or from focused sunlight. It can be a spark of electricity from an extension cord. When the hydrocarbon molecules reach 300 degrees Fahrenheit (150 degrees Celsius), the reaction begins.
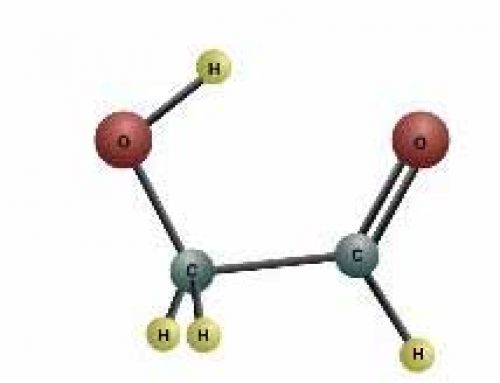
During the reaction, the molecules come apart and recombine into carbon, carbon dioxide, smoke, and water. But the reaction leaves a little energy left over, and that’s the heat and light of the fire.
Anything made of hydrocarbons, like wood, charcoal, alcohol, leaves, people, coal, oil, gas, or plastic, will burn. A few other things, like magnesium metal, will also burn. These are all combustion reactions. Combustion reactions are a kind of oxidation reaction – a kind that happens fast.
Learn by Doing – starting a fire
More about coal
A slower kind of oxidation reaction: rust
Another slow kind of oxidation reaction: digestion
Bibliography and further reading about fire: