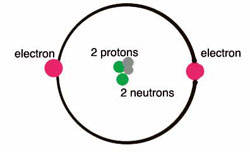
Diagram of a helium atom
Helium is a very simple atom.
Helium is a simple atom. The nucleus of a helium atom has two protons and two neutrons. Around the nucleus, there are two electrons. The only atom simpler than helium is hydrogen.
More about hydrogen
What are atoms made of?
All our chemistry articles
Stars and helium
There are helium atoms inside stars. The star makes helium by squashing four hydrogen atoms together into one new helium atom. So stars are made mainly of hydrogen and helium atoms. When a star runs out of hydrogen, it begins to turn helium atoms into carbon atoms instead.
What are stars made of?
Turning into carbon
Where on earth do we find helium?
But the helium people use on Earth mainly comes from mining underground gas pockets of helium. The helium gets underground when radioactive atoms like uranium (that are underground) decay and shoot off alpha particles, which are the same as helium atoms without their electrons. Still underground, the alpha particles find electrons and join up with them to become helium atoms.
What is uranium?
More about electrons
And radioactivity

Helium balloons
Helium is very light
Because helium atoms are small, they are very light. Like hydrogen, helium is lighter than air, so when you fill balloons with helium, they float.
Helium and explosions
But the main thing people use helium for is to keep things from exploding. Helium atoms are very stable – it’s hard to get them to combine with other atoms into molecules.
So if you are working with materials that might explode, you can do it more safely in a helium atmosphere. Welders, for example, use a lot of helium this way.
Will we run out of helium?
There’s a limited amount of helium on earth, and when we use it, it floats away into the atmosphere. We get it as a byproduct of natural gas. Radioactive decay makes more helium, but not as fast as we’re using it up. So one day, Earth may run out of helium, or at least have to spend more money to find new places where there’s still some helium left. But not right away.
Will we run out of helium?
Did you find out what you wanted to know about the helium atom? Let us know in the comments!
Learn by doing: Helium Balloons
Bibliography and further information about helium and atoms:



thanks
Since an alpha particle is the same as the nucleus of a helium atom it needs to capture two electrons to reach a stable state – presumably as a helium atom. This mental model may be too simple because one web site I have seen (which seems quite sensible) says an alpha’s “short flight knocks about 450,000 electrons out of the surrounding atoms”. Why is it so reactive? What am I misunderstanding?
It’s the same in terms of what it’s made of, but different in terms of how much energy it has, if I understand correctly. But I’m not a physicist; you should ask someone who is.
thanks
Glad we could help, Mr. Cunningham!