A diagram of a hydrogen atom
Hydrogen is the simplest kind of atom
Hydrogen is the simplest kind of atom, and in the very earliest days after the Big Bang hydrogen was the only kind of atom in the new Universe.
What are atoms?
What’s the Big Bang?
All our chemistry articles
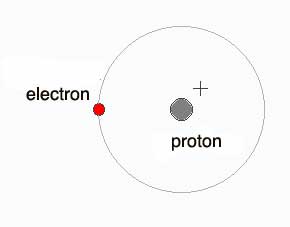
A hydrogen atom, like other atoms, has a nucleus. The nucleus of a hydrogen atom is made of just one proton. Around the nucleus, there is just one electron, which goes around and around the nucleus.
What is a nucleus?
And a proton?
Atoms and electrons

The Eagle Nebula
Hydrogen turns into other kinds of atoms
The first hydrogen atoms in space gradually grouped together into clumps called nebulae. When a nebula got dense enough, it formed a star.
More about stars
And about nebulae
What is gravity?
Inside these stars, there was a lot of heat and gravity. The strong gravity pressed the hydrogen atoms together, and four hydrogen atoms squashed together to form a new atom, with two protons, two neutrons, and two electrons. This new atom is helium. Hydrogen and helium atoms together make up almost all of the atoms in a star.
What is a neutron?
Nuclear fission
More about helium
Different types of stars
What is heat?
What is light?

The first men riding in a hydrogen balloon (in France, 1783 AD)
Hydrogen becomes planets
Not all the hydrogen stayed inside stars, though. Some of it began spinning around the outside of the star, orbiting the new star. This hydrogen mixed with other kinds of atoms. Gradually gravity pulled them together into planets. There are many hydrogen atoms on Earth (though most of them have joined up with other atoms to make molecules).
What is a planet?
What are molecules?
Hydrogen atom and water
Because hydrogen atoms are so simple, they are very light. They are lighter than air, so if you fill a balloon with hydrogen, it will float up to the sky (like a helium balloon that you can get at the store).
What is water made of?
What are hydrocarbons?
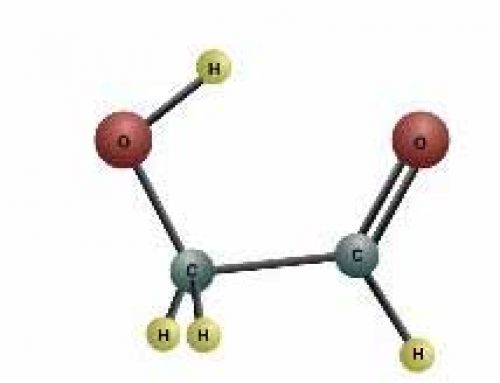
We get light and heat from hydrogen turning into helium. But there are two other important things about hydrogen for us. One is that two hydrogen atoms combine with one oxygen atom to make a molecule of water. All our water is made of hydrogen. And the other one is that hydrogen atoms combine with carbon atoms to make hydrocarbons, which all living things are made of.
Learn by doing: hydrogen
More about helium
Bibliography and further reading about hydrogen: