Diagram of a carbon atom
Where does carbon come from?
When a star has changed all of the hydrogen atoms into helium, it begins to convert the helium atoms into carbon atoms and oxygen atoms. All of the carbon in the universe was made inside stars.
More about stars
What is hydrogen?
Science projects with carbon
All our chemistry articles
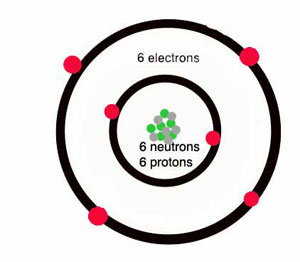
How many protons and neutrons are in carbon?
Carbon atoms are heavier – they have more mass – than helium or hydrogen, because they have six protons and (usually) six neutrons in the nucleus, and six electrons going around the outside.
What is a proton?
The strong nuclear force
What is mass?
How many shells does a carbon atom have?
The six electrons can’t all go around the nucleus at the same distance – only two electrons can fit in the inner shell. So some of the electrons have to go farther away from the nucleus. A carbon atom has two shells, with two electrons in the inner shell and four electrons in the outer shell.
What are electrons?
How do atoms work?
Why does carbon join up with other atoms?

Carbon soot coming out of a truck
But that outer shell could hold as many as eight electrons – it’s not full. That makes it very easy for carbon to combine with other atoms to make bigger molecules. A lot of carbon combines with oxygen to make carbon monoxide (one oxygen atom) or carbon dioxide (two oxygen atoms), for example.
What are molecules?
All about carbon dioxide
A carbon dioxide project
Examples of things made out of carbon
All living things on Earth are made mostly of hydrocarbons (molecules of hydrogen and carbon) and water (molecules of hydrogen and oxygen). Both plants and animals are about 18 per cent carbon. We’re all made out of the insides of stars!
What is a hydrocarbon?
How do plant and animals use carbon?
Plants get carbon by taking carbon dioxide out of the air and breaking off the oxygen atoms, and animals (including people) get carbon by eating plants or other animals. Animals recombine the carbon with oxygen to make carbon dioxide again, which is what you breathe out. And when plants and animals die, their bodies also gradually become carbon dioxide.
Fossil fuels and global warming
Most of what we use for fuel is made of carbon, too. Charcoal, coal, gasoline, oil, and wood are all made of hydrocarbons. They’re the remains of dead plants – that’s why they’re made of hydrocarbons – that didn’t decay all the way to turn back into carbon dioxide. When we burn them, they do release that energy.
More about global warming
What is oil?
History of coal
Combustion reactions
In the picture you can see leftover carbon dust pouring out of this truck that burns diesel fuel. It’s this carbon pouring into the air and combining with oxygen to make extra carbon dioxide that’s causing global warming.
Learn by doing: candles and car exhaust
What is carbon dioxide?
More about charcoal
More about coal
Bibliography and further reading about carbon atoms:




