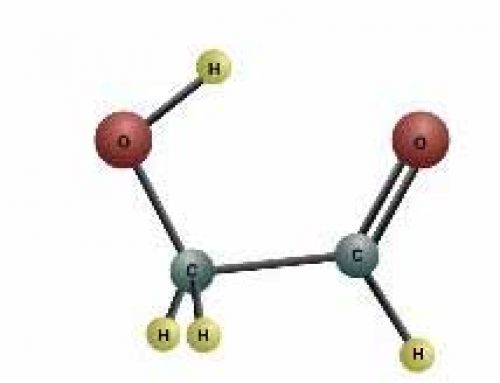
Covalent bonds: two hydrogen molecules bonding to an oxygen molecule
Covalent bonds stick atoms together
When two atoms come near each other, sometimes they stick together to make a molecule. One way they can stick together is by covalent bonding.
Ionic bonding
More about molecules
What are atoms?
All our chemistry articles
Are covalent bonds stronger than ionic bonds?
In covalent bonding, the atoms are unstable because their outer rings of electrons aren’t filled up. By sharing electrons with other atoms, these atoms can fill up their outer rings and become stable. In water, for instance, the oxygen atom needs two more electrons to be stable, and the hydrogen atoms each need one.
What are electrons?
More about water
When they get together, the oxygen atom shares one electron with each of the hydrogen atoms, and the hydrogen atoms each share one electron with the oxygen atom.
More about space
Evolution of cells
That’s why the question of whether covalent bonding is stronger than ionic bonding is hard: because in space, in a vacuum, ionic bonds are stronger, but in real life, we’re often talking about covalent bonds in living cells, and so they’re in water. In water, covalent bonding is stronger than ionic bonding.
Covalent bonds make liquids, gases, and metals
Molecules that join with covalent bonds aren’t very much attracted to each other (unlike with ionic bonding), so they move freely around each other. That means that most molecules that form covalent bonds make either liquids or gases, like water and carbon dioxide.
More about carbon dioxide
The main exception is metals, which hold together using covalent bonding but are still solids. That’s why metals are so flexible and easy to melt so you can make them into different shapes.
More about metals
Did you find out what you wanted to know about covalent bonding? Let us know in the comments!
Learn by doing: try bending copper wire into sculptures
Ionic bonding
Bibliography and further reading about covalent bonding: