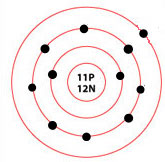
Diagram of a sodium atom
Sodium is an atom that has 11 protons and 12 neutrons in its nucleus and 11 electrons circling around its nucleus. Like other light atoms such as carbon, sodium forms inside of stars that are beginning to run out of fuel, and it scatters all over space when that star explodes in a supernova.

Sodium is soft, and you can cut it with a knife.
Because it has only one electron in its outer ring, sodium joins up easily with other atoms to make molecules. You never find it by itself in nature. Sodium joins especially easily with chlorine, because sodium needs to lose an electron to be stable, and chlorine needs to gain an electron to be stable. So once sodium and chlorine join together, it is hard to break them apart. This combination of sodium and chlorine is salt.
There’s a lot of sodium on Earth. Most of the sodium on Earth is mixed with chlorine to make salt, and it is dissolved in water in our oceans.
Learn by doing – Sodium
More about salt
Salt in human history
Bibliography and further reading about atoms: