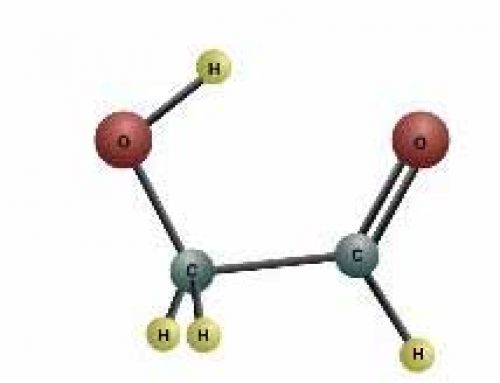
Chalk has calcium in it
Take a piece of regular white chalk, like for writing on a blackboard. Make sure the chalk is calcium carbonate like the kind in the picture. Some kinds of chalk are made of plaster of paris. You don’t want those.
What is calcium?
More chemistry projects
A chemical reaction
Drop the chalk in a cup of vinegar or lemon juice. Vinegar is made of water and acetic acid, a kind of hydrocarbon. It should fizz up. That’s because the oxygen in the acetic acid combines with the calcium carbonate. The bubbles are carbon dioxide. Carbon dioxide forms when the oxygen from the acetic acid joins with the carbon from the calcium carbonate.
What is oxygen?
What are hydrocarbons?
And carbon dioxide?
What is left over?

A robin’s nest with three eggs in it
When the chalk is done fizzing, you should see a layer of little particles of calcium acetate at the bottom of the cup. This is the calcium left over from the calcium carbonate, together with the hydrogen left over from the acetic acid.
What is hydrogen?
Experiments with eggs and bones
How did eggs evolve?
More about bones
Frog skeleton (thanks to Bone Clones)
To see that bones are also made of calcium carbonate, try dropping a scrubbed chicken bone in vinegar. How about somebody’s baby tooth? Or a piece of marble?
What if you add baking soda?
By adding baking soda back to the calcium, you can make it back into calcium carbonate. Baking soda is made of sodium bicarbonate – molecules of sodium combined with carbon.
More about sodium
And carbon
Now add baking soda and water to the dissolved calcium. The carbon from the baking soda will combine with the calcium to make calcium carbonate again. You should see white particles appear in the liquid.
More about calcium atoms
More chemistry projects
Bibliography and further reading about chemistry projects: